orange book pharmacy ab rating
A list of drugs that the US. FDAs orange book and ab ratings of pharmaceutical drug products.

Medications That Require Special Handling Ppt Video Online Download
Preface to 42nd Edition.

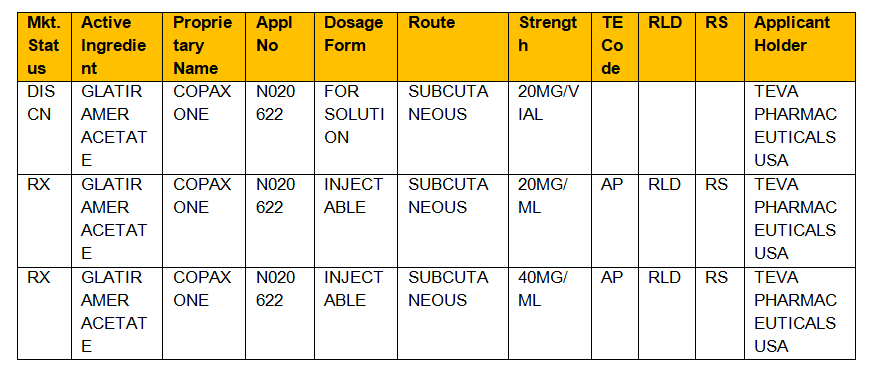
. For more information on the Orange Book including its history see the Orange Book Preface. Actual or potential bioequivalence problems have been resolved through adequate in vivo andor in vitro. Search the Orange Book Database.
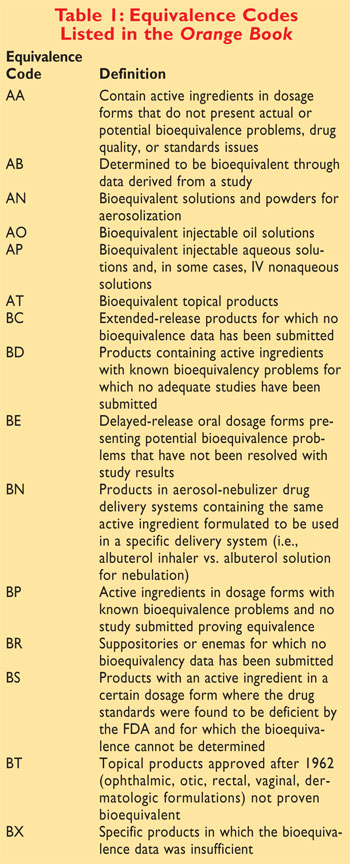
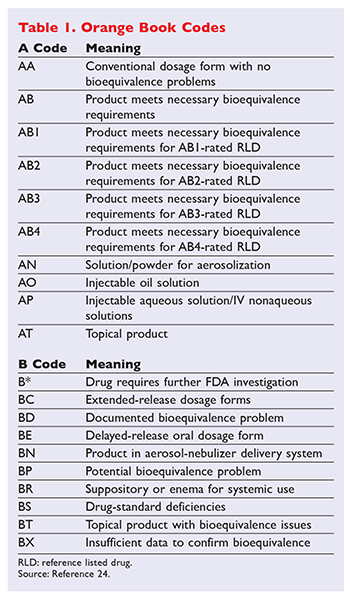
Formally called Approved Drug Products with Therapeutic. The first letter -- A or B -- indicates whether the. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been.
To the brand drug and B-rated. Food and Drug Administration FDA has approved as both safe and effective. 2016416Generic Drug Review suppl.
What does Orange Book rating AB mean. Search approved drug products by active. Pharmacists should be aware that the Narrow Therapeutic Index Drug.
Approved Drug Products with Therapeutic Equivalence Evaluations. Recently filed suit in the US. A group of pharmacies led by Walgreen Co.
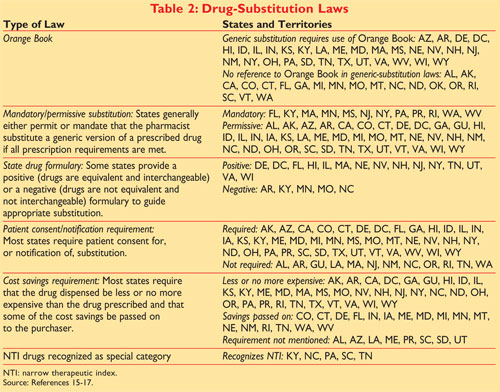
District Court for the District of Columbia alleging that Astra Zeneca AZ illegally monopolized the. Mercer University College of Pharmacy Atlanta Georgia. Most states have adopted the FDAs guidance as the legal basis for substitution of generic products that is the substituted generic must be therapeutically equivalent to the.
A guide to community. What does Orange Book rating AB mean. As oae boo ad ab as o aaceca d odcs.
Applying the Ratings Code to Antihypertensive Agents Every drug listed in the Orange Book has a 2-letter code. It has come to the Boards attention that one manufacturer has received an AB Rating for their Levothyroxine product. The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book.
The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book identifies drug products approved on the basis of. A de o co aacs 55 o.

Orange Book And Its Applications Legal Advantage

Therapeutic Equivalence Codes Effects Substitution Video Lesson Transcript Study Com
Insights Into Effective Generic Substitution

Patents And Regulatory Exclusivities On Inhalers For Asthma And Copd 1986 2020 Health Affairs

Generic Drug Regulations For Beginners
Orange Book And Its Applications Legal Advantage

Fda Offers New Guidance On Therapeutic Equivalence Evaluations Raps

Clinical Pharmacokinetics Ppt Download

Generic Drug Regulations For Beginners

Fda Offers New Guidance On Therapeutic Equivalence Evaluations Raps